ASTM publishes E2834 standard defining Nanoparticle Tracking Analysis
Salisbury, UK, 3rd July 2012: NanoSight is delighted to welcome publication of American Society of Testing Materials ASTM E2834 - Standard Guide for Measurement of Particle Size Distribution of Nanomaterials in Suspension by Nanoparticle Tracking Analysis (NTA).
This document details application of Nanoparticle Tracking Analysis (NTA) to measurement of particle size distributions for suspended particles from ~10nm to the onset of sedimentation.
Duncan Griffiths, NanoSight's USA West Sales Manager comments "This document has taken almost three years to develop. It provides a rigorous review of the core science driving Brownian motion and the derived parameters of mean, mode, percentile values and concentration. The scrutiny of demanding third-party experts has been fundamental in producing a thorough appraisal of NTA. The result is a meticulous presentation of science and methodology. We are delighted".
The UK's National Physical Laboratory (NPL) has seen NanoSight develop NTA since its early days more than five years ago. Helen Sharman from NPL comments: "NPL has been working on the characterization of nanomaterials for many years, in-part collaborating with NanoSight. The nanomaterials team has followed the development of the NTA technique with great interest due to its unique capability in the area of nanometrology. As the UK's national measurement institute, NPL recognizes the importance of this standard and the capability of the NanoSight instrumentation to provide consistent, precise and comparable data."
NanoSight CEO Jeremy Warren adds "Whilst this standard is a milestone, it is also a building block. Many NanoSight users have requested this definition of the technique as they present data in research papers and to regulatory bodies for product approvals and clinical trials. We will now move to provide industry with test methods for specific materials to help lock down SOPs. The timing of this publication is helpful as the European Commission move to consider methodologies to address the characterization challenges of their recently-published definition of nanomaterials. Here we see a significant role for NTA's unique nanoparticle counting capability".
The Standard is available from ASTM at
http://www.astm.org/Standards/E2834.htm
To learn more about particle characterization using NanoSight's unique nanoparticle tracking analysis solutions, visit
www.nanosight.com and register to receive the next issue of NanoTrail, the company's electronic newsletter.
Attachment
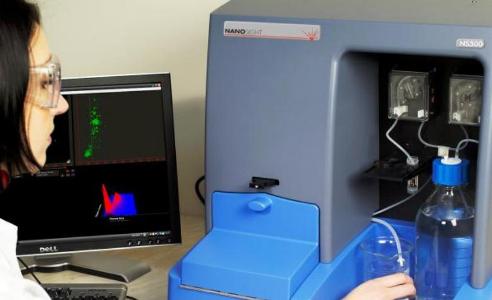 Nanoparticle Tracking Analysis software run on the NanoSight NS500
Nanoparticle Tracking Analysis software run on the NanoSight NS500
For a high resolution copy of the image, either right click to download or contact Jezz Leckenby at Talking Science.
About NanoSight:
NanoSight delivers the world's most versatile and proven multi-parameter nanoparticle analysis in a single instrument.
NanoSight's "Nanoparticle Tracking Analysis" (NTA) detects and visualizes populations of nanoparticles in liquids down to 10 nm, dependent on material, and measures the size of
each particle from direct observations of diffusion. Additionally, NanoSight measures concentration and a fluorescence mode differentiates suitably-labelled particles within
complex background suspensions. Zeta potential measurements are similarly particle-specific. It is this particle-by-particle methodology that takes NTA beyond traditional light
scattering and other ensemble techniques in providing high-resolution particle size distributions and validates data with information-rich video files of the particles moving
under Brownian motion.
This simultaneous multiparameter characterization matches the demands of complex biological systems, hence its wide application in development of drug delivery systems, of
viral vaccines, and in nanotoxicology. This real-time data gives insight into the kinetics of protein aggregation and other time-dependent phenomena in a qualitative and quantitative
manner. NanoSight has a growing role in biodiagnostics, being proven in detection and speciation of nanovesicles (exosomes) and microvesicles.
NanoSight has installed approaching 500 systems worldwide with users including BASF, GlaxoSmithKline, Merck, Novartis, Pfizer, Proctor and Gamble, Roche and Unilever together with the most eminent universities and research institutes. NanoSight's technology is validated by 450+ third party papers citing NanoSight results and by the ASTM Standard E2834, consolidating NanoSight's leadership position in nanoparticle characterization. For more information, visit www.nanosight.com.
Want to know more information, please contact phone number:
| 香港 Hongkong |
北京BeiJing |
上海ShangHai |
T:(852)27556578
F:(852)27554549
info@anp.com.hk |
T:(010)62074835
F:(010)62077434
bj@anp.com.hk |
T:(021)55233800
F:(021)55233811
sh@anp.com.hk |
| 广州GuangZhou |
深圳ShenZhen |
成都ChenDu |
T:(020)66836899
F:(020)66830898
gz@anp.com.hk |
T:(0755)82202428
F:(0755)82202438
sz@anp.com.hk |
T:(028)85216348
F:(028)85216348
cd@anp.com.hk |
| 武汉WuHan |
台湾TaiWan |
新加坡Singapore |
T:(029)87591299
F:(029)87591239
wh@anp.com.hk |
T:(886)22953164 6
F:(886)22953986 3
tw@anp.com.hk |
T:(65)62413359
M:(65)62410909
sg@anp.com.hk |